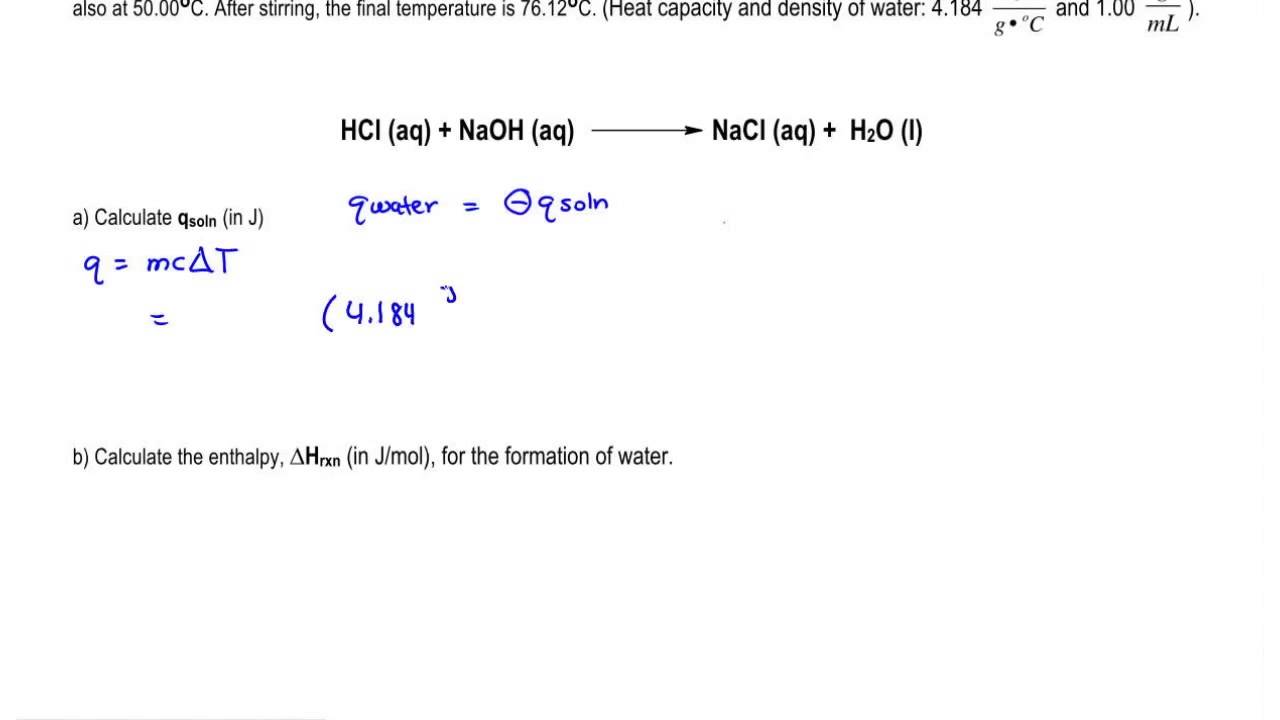
How to Find Heat Absorbed by Solution
Q m c s T M - T i m L f m c l T B - T M m L V m c g T f - T B Q - - -. C 418 J g-1K-1.
Calculating The Heat Of The Solution Youtube
The formula of the heat of solution is expressed as ΔH water mass water ΔT water specific heat water.
. The Calorimetry Formula Q heat evolved equal to heat absorbed heat released in. Q amount of energy released or absorbed. In part I of the graph the substance is in solid state.
Q SOL 397 Jg C o 107 g 475 C o 2020 J actually only 3 significant figures are justified But some of the liberated heat went to raising the temperature of the calorimeter. Made by faculty at the University of Colorado Bould. Substitute the solutions mass m temperature change delta T and specific heat c into the equation Q c x m x delta T where Q is the heat absorbed by the solution.
Divide the change in enthalpy of the solution by the number of moles of KCl to determine the molar heat of solution of KCl. Q m Cg ΔT. Assume the specific heat of the solution is the same as the specific heat of water 4184 Jgx0C and that the mass of water in grams volume of.
186 joules per gram Celsius. When solid or gas is dissolved in the solvent the heat is absorbed. In part III of the graph the substance is in liquid state.
Calculate the amount of heat in Joules J absorbed or liberated per gram of salt and determine whether the process is exothermic or endothermic. Calculate moles of solute. The initial temperature of this solvent is recorded T i.
Assume the denisty of thefinal solutions is 103gmL and the specific heat is 418 JgC. Q mass in grams of reactant a mass in grams of reactant b 4184 T final - T initial. SolvationThe process of attraction and association of molecules of a solvent with molecules or ions of a solute.
Solution highest or lowest temperature attained. 234 x 32 - 87 J -5148 J. The heat solution is measured in terms of a calorimeter.
Mass water sample mass. 387 x 32 - 27 J 774 J. We must use the formula Q m L f to calculate the heat absorbed by it during this stage.
And the quantity of heat transferred to the water in sections 2 and 4 is related to the mass of the sample and the heat of fusion and vaporization by the formulae Q mΔH fusion section 2 and Q mΔH vaporization section 4. Find the solutions specific heat on a chart or use the specific heat of water which is 4. The process involves drop in temperature which indicates that dissolution of salt absorbed heat from the system.
17131 CaCl 2 s Ca 2 a q 2 Cl a q 828 kJ. Heat of solutionThe enthalpy change associated with the dissolution of a substance in a solvent at constant pressure resulting in infinite dilution. Assuming that all heat transfer was between the rebar and the water with no heat lost to the surroundings then heat given off by rebar heat taken in by water or.
Substitute the solutions mass m temperature change delta T and specific heat c into the equation Q c x m x delta T where Q is the heat absorbed by the solution. C m ΔTrebar c m ΔTwater. The heat capacity of the calorimeter must be determined experimentally.
Heat energy absorbed by the substance calculations. You should be multiplying 365g by the temperature change and heat capacity. Ideally the components of the calorimeter would absorb no heat but the components of the calorimeters always absorb some energy from the system.
Δ H heat change. Q rxn -heat absorbed by solution heat absorbed bycolorimeter q rxn -grams of solution x specific heat of solutionx ΔT solution C cal xΔT solution where ΔT solution T mix-T initial for each reaction. In part II of the graph the substance is melting.
An accurately known quantity of water the solvent is placed in a well insulated vessel eg a polystyrene foam. The heat absorbed or transferred by each substance is calculated using the equation. From the given heat capacity of the solution and the measured temperature change we can calculate the amout of heat absorbed by the solution.
The total mass of the solution is 150g 350g 365g. Since heat absorbed by the salt is equal to the heat lost by water We have the formula Q mCΔT. Many hot packs use calcium chloride which releases heat when it dissolves according to the equation below.
Formula of Heat of Solution. Then you need to consider how many moles 150g KCl is. ΔT 25 21 4 K.
Q absorbed 40 x 10 -3. The heat of solution also referred to the enthalpy. We must use the formula l.
To calculate the enthalpy of solution heat of solution using experimental data. An accurately known quantity of the solid solute is added the vessel is sealed with a lid. For example if a solution of salt water has a mass of 100 g a temperature change of 45 degrees and a specific heat of approximately 4186 joules per gram Celsius you would set up the following.
We must use the formula s stands for solid to calculate the heat absorbed by it during this stage. So now we will make an effort to calculate the quantity of heat required to change 500 grams of water from the solid state at -200C to the gaseous state at. Heat Absorbed or Released Calculator Results detailed calculations and formula below The heat energy absorbed or released by a substance with or without change of state is J.
Q 100 418 4. Therefore Q -1672J. N m M.
Calculate the amount of heat added to a system in which 45 g of carbon reacts in an endothermic reaction. Grams 100 Tmix 347 Tinitial 23 C for HCl. Typically the calculation for heat released or absorbed q for the reaction of aqueous solutions is measured in units of joules J.
Since we know how heat is related to other measurable quantities we have. How much heat is a reaction producing or absorbing. Q absorbed 225 x 10 -3.
4186 x 32 -. This process is known as heat dissolution or heat solution. Amount of energy released or absorbed is calculated.
The molar heat of solution Δ H soln of a substance is the heat absorbed or released when one mole of the substance is dissolved in water. Qrebar qwater. Q yielded 400 x 10 -3.
The heat capacity of the calorimeter is the quantity of heat absorbed by the calorimeter for each 1C rise in temperature. Given parameters are m 100g. ΔHsoln q n.
Amount of energy heat released or absorbed per mole of solute is calculated.

Heat Of Solution Chemistry For Non Majors
How To Find How Much Heat Has Been Absorbed Quora

No comments for "How to Find Heat Absorbed by Solution"
Post a Comment